Latest Ground Breaking Innovations in the Treatment of Cancer
Posted by Phil Heler on February 5, 2023For the first time in 2022 a new exciting technology called CART Cell Therapy was used with a modified gene editing technique called Base Editing to treat cancer. This is a fast-moving research area with massive potential. It has generated huge excitement among researchers and oncologists. So how does it work?
Looking back, 2022 was a year of exciting scientific innovation. Generating nuclear fusion on an industrial scale could solve our future energy demand problems. This is the same clean energy that drives the stars, such as our sun, by forcing hydrogen atoms together.
Fusing atoms together in a controlled way releases nearly four million times more energy than a chemical reaction such as the burning of coal, oil or gas.
It is a scientific holy grail. On 11th of December at the Lawrence Livermore National Laboratory (LLNL) in California, for the first time a controlled fusion reaction generated more power than was required to run it. The experiment is a major step towards commercial fusion power, but there is still a vast engineering effort needed to increase efficiency and reduce cost. Innovation in healthcare also scaled new heights.
Up until now, the foundations of cancer treatment have been surgery, chemotherapy, and radiation therapy. While these methods are still key pathways for treatment, there are exciting new categories of treatment that have recently helped reshuffle clinical options.
Over the last 10 years immunotherapies that help strengthen our immune systems have become another key asset. These therapies amplify our immune system to the extent that it can seek out, shrink and, in some cases, eliminate even advanced tumours.
One particular form of immunotherapy trialled in 2022 was a form of CART Cell Therapy that used a modified gene editing technique called Base Editing. This is a fast-moving research area with massive potential. It has generated huge excitement among researchers and oncologists. The approach may have the potential to eradicate very advanced leukaemia and lymphomas. So how does it work?
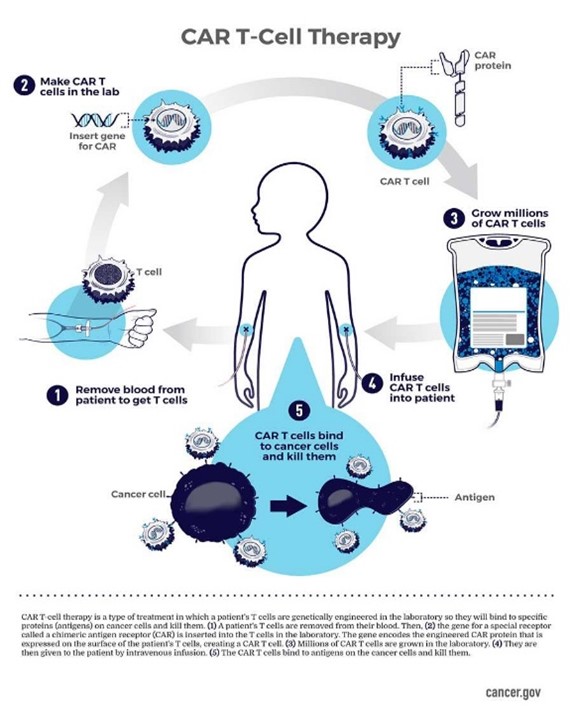
CAR T-cells are literally the equivalent of giving patients a living drug. They work by revamping part of our immune system called T lymphocytes. T lymphocytes play a significant part in our immune response. CAR T-cell therapies are made by collecting T lymphocytes from patients (or other donors).
These are then re-engineered in the laboratory. These changed T-cells are designed to have new receptors called chimeric antigen receptors (or CAR T-cells). The cells are grown in large numbers in the laboratory and given to cancer patients. The CAR T-cells are designed to bind to certain proteins on cancer cells.
This helps the T-cells identify and eradicate cancer cells that have the specific protein that the receptor is designed to bind to. CAR T-cells will continue to multiply in the patient’s body and, with guidance from their engineered receptor, continue to recognize and kill any cancer cells that continue to harbour the target antigen on their surfaces.
Leukaemia, as many of us know, is caused by our own immune cells from our bone marrow proliferating and multiplying beyond control. The traditional approach is treatment which destroys all our bone marrow cells using chemotherapy and then replacing the bone marrow with a transplant. This is successful in most cases. If it fails, CAR-T therapy has been available as an option but only in 2022 has it been combined with a Base Editing capability.
Until now CAR-T therapy has depended on a gene editing technique called CRISPR to make new receptors on T lymphocytes. CRISPR as a process began in the 1980s when researchers in Japan noticed a peculiar series of frequently repeating sequences in the DNA of bacteria, separated by what appeared to be random bits that were later called ‘spacers’. These characteristic sets of repeats and spacers became known as ‘clustered regularly interspaced short palindromic repeats’, or CRISPR.
Nobody really understood what these were until 2005 when it was noticed that these apparently random repeats of CRISPRs in the DNA of bacterial cells matched parts of DNA present in many viruses.
This match was no coincidence. In bacteria, these CRISPR repeats are copied from the DNA of viruses that have previously attacked the cell. They therefore act as a bank of memories, which enables bacteria to recognize the viruses and fight off future attacks thus forming an integral part of their immune system. In this way the cell’s immune database can be kept up to date.
This process was detailed in 2014 by Nobel Prize winners in Chemistry Jennifer Doudna and Emmanuelle Charpentier in the journal Science. Crucially they realised that this system could potentially be a remarkably simple yet extremely powerful gene-editing tool that could have widespread applications.
This has since been realised. They proved that the CRISPR system worked flawlessly even in human cells where it could be used to interrogate and cleave to DNA thus acting as a molecular pair of scissors. The true beauty of the system lay in its simplicity.
Gene editing using CRISPR traditionally involves cutting DNA strands and relying on cellular machinery to re-join the ends. However, when lots of cuts are made at once, cells sometimes die.
Unfortunately, even if they do survive mistakes are often made; the wrong ends can be put back together, leading to major mutations. The more gene edits that are made, the more likely this is to occur. So, the system is prone to error. As the cell repairs the break, random bases can be inserted or deleted (indels), altering the gene sequence. Large chromosomal segments might even be deleted or rearranged.
In 2022 a modified form of the CRISPR gene-editing protein was used that does not involve cutting or breaking the DNA backbone. Instead the new technique involved changing one DNA letter to another, a technique known as base editing. The human genome is made up of just four simple units called bases, these are found in repeating units in our DNA: adenine (A), cytosine (C), guanine (G), and thymine (T).
These bases are quite literally the letters that spell our genetic code and hold precious instructions of life. The genome comprises more than 3 billion of these base pairs in two double strands of DNA that form a helix; the sequence of these bases encodes our genes.
Even one mistake in a single letter of a gene, known as a point mutation, can result in disease. Base editing allows scientists to zoom to a precise part of the genetic code and then alter the molecular structure of just one base, converting it into another and changing the genetic instructions.
Base editing was invented in 2016 by scientists who made enzymes from scratch. These were made by setting up a system in which bacteria had to evolve such an enzyme to survive. Using this method they made two enzymes, or base editors, called BE3 and ABE7. BE3 can make two changes by changing C to T and G to A. ABE7 meanwhile turns A into G and T into C.
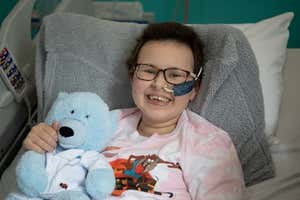
Last year a large team of doctors and scientists used this tool for the first time to engineer a new type of T-cell that was capable of hunting down and killing a young patient’s cancerous T lymphocyte cells. The 13-year-old girl, called Alyssa, had not responded to any other treatment.
Alyssa, from Leicester, was diagnosed with T-cell acute lymphoblastic leukaemia in May 2022 with little hope of a cure. As part of a trial, she received a dose of immune cells from a donor that had been modified to attack her cancer. Just twenty-eight days later, investigations revealed she was in remission.
Rather than using her own T lymphocytes they used healthy T-cells that came from a donor and set about modifying them. The first base edit disabled the T-cells targeting mechanism so they would not cause damage to Alyssa’s body. The second base edit removed a chemical marking, called CD7, which is on all T-cells, so that it would not destroy itself.
The third edit was an invisibility cloak that prevented the cells being killed by a chemotherapy drug. The final stage of genetic modification instructed the T-cells to go hunting for anything with the CD7 marking on it so that it would destroy every T-cell in her body – including the cancerous ones. This is why the CD7 obviously had to be removed from the modified cells.
Genetic manipulation is a very fast-moving area of science with enormous potential across a range of diseases. Alyssa is just the first of 10 people to be given the drug as part of a clinical trial. Dr Robert Chiesa, from the bone-marrow transplant department at Great Ormond Street Hospital, said: ‘It is extremely exciting. Obviously, this is a new field in medicine and it’s fascinating that we can redirect the immune system to fight cancer.’
But this only scratches the surface of what base editing could achieve. Base editing — the introduction of a single changed letter in the genetic code in the DNA or RNA in living cells — could solve around half of known pathogenic genetic diseases through alteration. Watch this space!!!